
News

News
Recently, Baowen Zhou’s group at the School of Mechanical Engineering in Shanghai Jiao Tong University has published a research article in Science Bulletin entitled 'An Integrated Photocatalytic Redox Architecture for Simultaneous Overall Conversion of CO2 and H2O toward CH4 and H2O2'. This paper presents a novel integrated photocatalytic redox architecture that uses sunlight to convert carbon dioxide (CO2) and water (H2O) into green methane (CH4) and hydrogen peroxide (H2O2).
Renewable synthetic fuels are crucial for decarbonizing energy and power sectors in the future. Methane, a widely used chemical fuel, has well-established infrastructures for storage, transportation, and utilization. However, it is primarily derived from non-renewable fossil resources such as natural gas. H2O2 is an important fine chemical with extensive applications in environmental remediation, organic synthesis, pharmaceuticals, and electronics industries. Traditional processes for H2O2 production, such as the anthraquinone method, are complex and environment-unfriendly. Using solar energy to convert CO2 and water into green methane and hydrogen peroxide via artificial photosynthesis is ideal for producing carbon-neutral fuels and high-value chemicals.
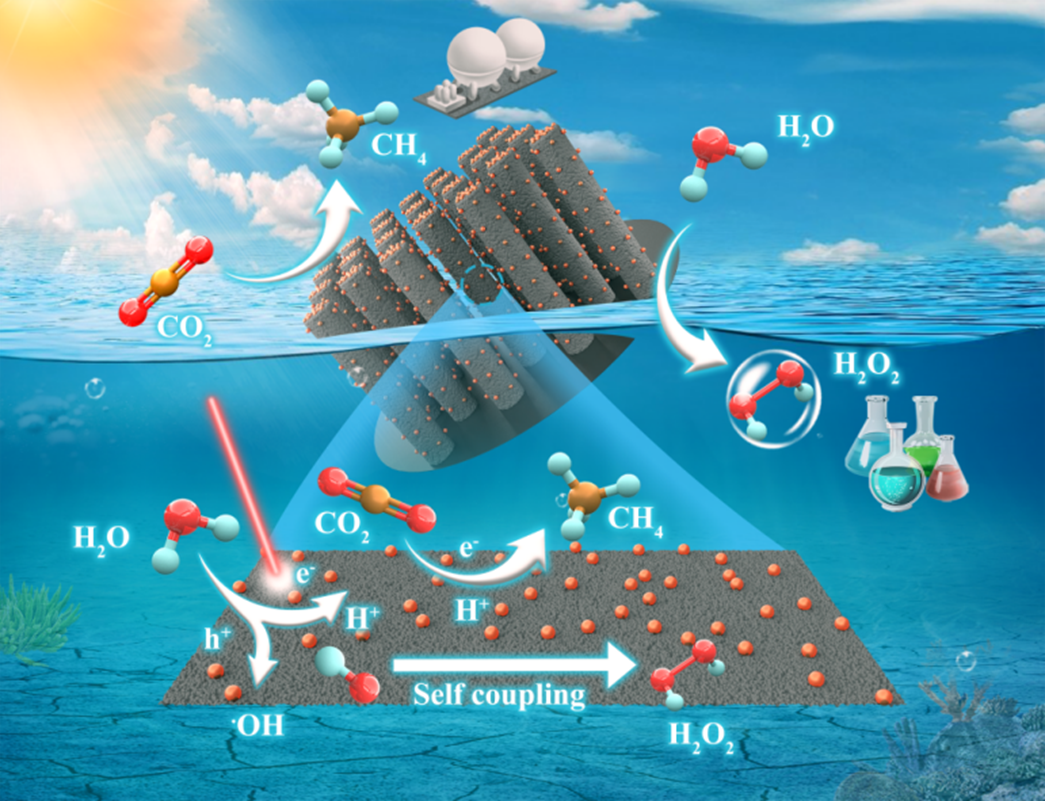
The schematic diagram of the integrated photocatalytic redox architecture based on Zn/GaN NWs/Si for simultaneous production CH4 and H2O2 from CO2 and H2O
The study demonstrates a novel integrated photocatalytic redox architecture by coupling zinc nanoparticles (Zn NPs) with gallium nitride (GaN) nanowire arrays on a silicon substrate (Zn NPs/GaN NWs/Si). Thanks to its excellent optoelectronic and catalytic properties, this architecture, under concentrated light irradiation, operates without sacrificial agents or other external energies. It efficiently converts CO2 and H2O into CH4 and H2O2 at a CH4 production rate of 189 mmol/g/hr with a selectivity of 93.6%, along with a H2O2 production rate of 25 mmol/g/hr.
In-situ spectroscopies, isotope-labelling experiments, and density functional theory (DFT) calculations elucidated the reaction mechanism for the simultaneous conversion of CO2 and H2O into CH4 and H2O2. It is found that Zn NPs significantly lowered the energy barrier for CO2 reduction on the GaN nanowire surface, while increasing the energy barrier for the hydrogen evolution reaction (HER), thereby promoting selective CH4 production from CO2 reduction and inhibiting the HER. Additionally, Zn nanoparticles weakened the adsorption strength of *OH on the GaN nanowire surface, reducing its coupling reaction energy barrier and facilitating H2O2 formation. The first author of this research article, Dr. Muhammad Salman Nasir said “This integrated architecture enables automatic phase separation of CH4 and H2O2, along with the high economic value of H2O2, making this process more suitable for industrial applications”. What is more, this integrated architecture in a wafer panel configuration is highly favourable for the distributed use of solar energy for artificial photosynthesis of green fuels and high-value chemicals with the only inputs of water and carbon dioxide.
Paper Link:https://www.sciencedirect.com/science/article/pii/S2095927324008272

Shanghai Jiao Tong University
Address: 800 Dongchuan Road, Shanghai
200240